NEURO VITAMIN COMPLEX
The Complete Guide to B6, B9 & B12 for Cognitive Health: Neurotransmitter Synthesis, Homocysteine Metabolism & the VITACOG Trial
Quick Answer
The neuro vitamin complex (B6, B9, B12) reduces brain atrophy by up to 53% in older adults with mild cognitive impairment and elevated homocysteine. The landmark VITACOG trial showed 30% slower brain shrinkage over 24 months using folic acid 800μg, B12 500μg, and B6 20mg daily. Benefits depend heavily on omega-3 status—without adequate DHA, B vitamins show minimal cognitive effect.
Table of Contents
Key Takeaways
- B6, B9, B12 reduced brain atrophy by 30% overall in VITACOG (53% in high-homocysteine group)
- Elevated homocysteine (>11μmol/L) is neurotoxic via NMDA receptor activation
- Benefits require adequate omega-3 DHA—no effect in lowest omega-3 tertile
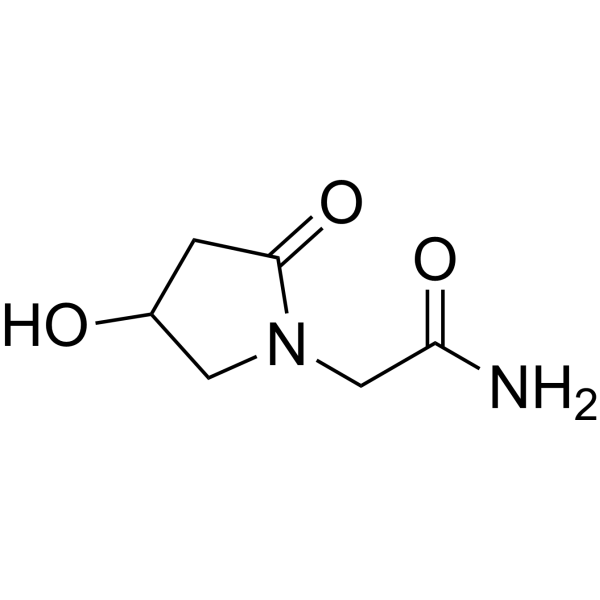
- Active forms (5-MTHF, methylcobalamin, P5P) bypass genetic polymorphisms
- ~40% of population carries MTHFR variants affecting folate metabolism
- B6 doses above 12mg/day carry peripheral neuropathy risk (EFSA 2023)
- Minimum 18-24 months supplementation needed for structural brain benefits
- Target populations: MCI patients, metformin users, vegans, PPI users